This kit targets highly conserved regions of the dengue virus, designing specific primers and probes that bind specifically to the target sequence. Under the action of reverse transcriptase and hot-start Taq DNA polymerase, this fragment is specifically amplified. During amplification, the fluorescent probe is hydrolyzed, producing fluorescence. The detection system includes dUTP-UDG enzyme contamination prevention measures, thoroughly degrading potential product contamination to avoid false-positive results. Additionally, an internal control is set for a highly conserved region of the human genome, monitoring the entire process of sample collection, transportation, nucleic acid extraction, and PCR amplification. This prevents false-negative results and ensures the effectiveness of the entire process.
Advantages: This kit qualitatively detects dengue virus nucleic acid for clinical diagnosis. It includes contamination prevention to avoid false positives and a human internal control to prevent false negatives. Results are available in 45 minutes.
High Applicability: Suitable for human serum and/or plasma.
High Sensitivity: Detects down to 500 copies/mL, tested with three different reagent batches.
High Specificity: No cross-reactivity with common pathogens like hepatitis B and C, influenza A and B, herpes simplex, encephalitis, and cytomegalovirus.
High Accuracy: Quickly detects dengue virus with 100% accuracy in positive and negative reference samples.
Clinical Diagnosis
Public Health Surveillance
Research Laboratories
Border and Entry Screening
Field Surveys
1. Accuracy: Positive reference samples (P1-P4) and negative reference samples (N1-N8) provided by Hangzhou Bioer Technology Co., Ltd. were reconstituted as required and then extracted using the Bioer extraction reagent BSC86S1E (Zhejiang Medical Device Registration No. 20200872) for subsequent testing.
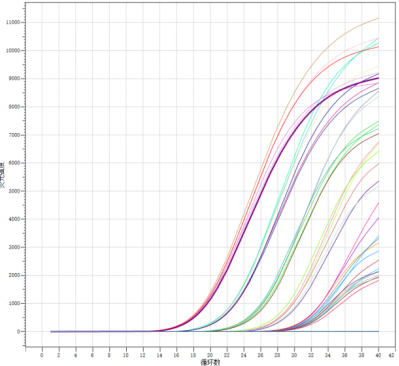
The results indicate accurate detection of dengue virus nucleic acid. The testing of company reference samples shows a 100% positive agreement rate and a 100% negative agreement rate.
2. Precision: The precision reference samples (J1-J2) of the dengue fever virus nucleic acid detection kit (fluorescent PCR method) were reconstituted according to the instructions for use. After extraction with the Bioer extraction reagent BSC86S1E (Zhejiang Medical Device Registration No. 20200872), testing was conducted using three batches of reagents, with each sample tested 10 times.
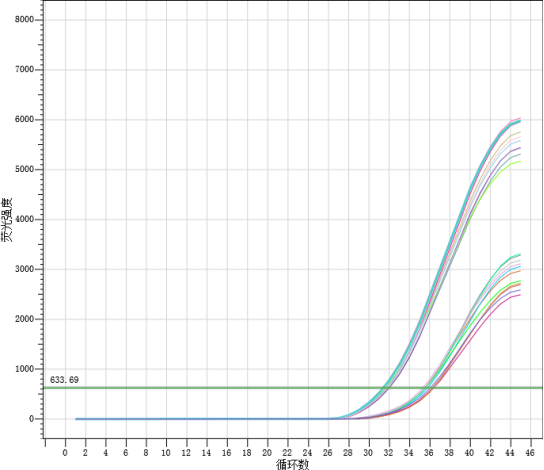
The results indicate that the inter-batch and intra-batch precision coefficients of variation for the three batches of reagents are all less than 5%, demonstrating good reagent precision.
-20 ± 5 ℃
| Cat# | Product Name | Packing size |
| BSJ43M1 | Dengue Virus Nucleic Acid Detection Kit (Fluorescent PCR) | 48T |
| BSJ43L1 | Dengue Virus Nucleic Acid Detection Kit (Fluorescent PCR) | 96T |

