Triple certification of nmpa, CE and FDA
On March 22, 2021, the sample preservation solution bsc82 of Hangzhou bioer Technology Co., Ltd. obtained FDA certification. Up to now, the product has obtained triple certification of nmpa, CE and FDA, and the product quality has been highly recognized.
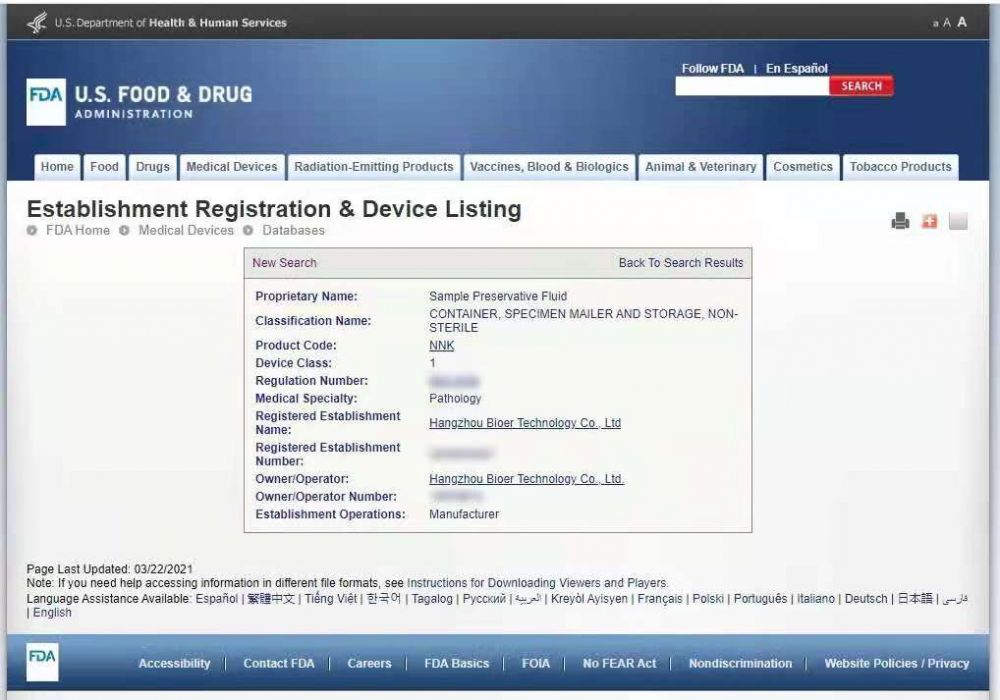
FDA certified

CE certified
Product introduction
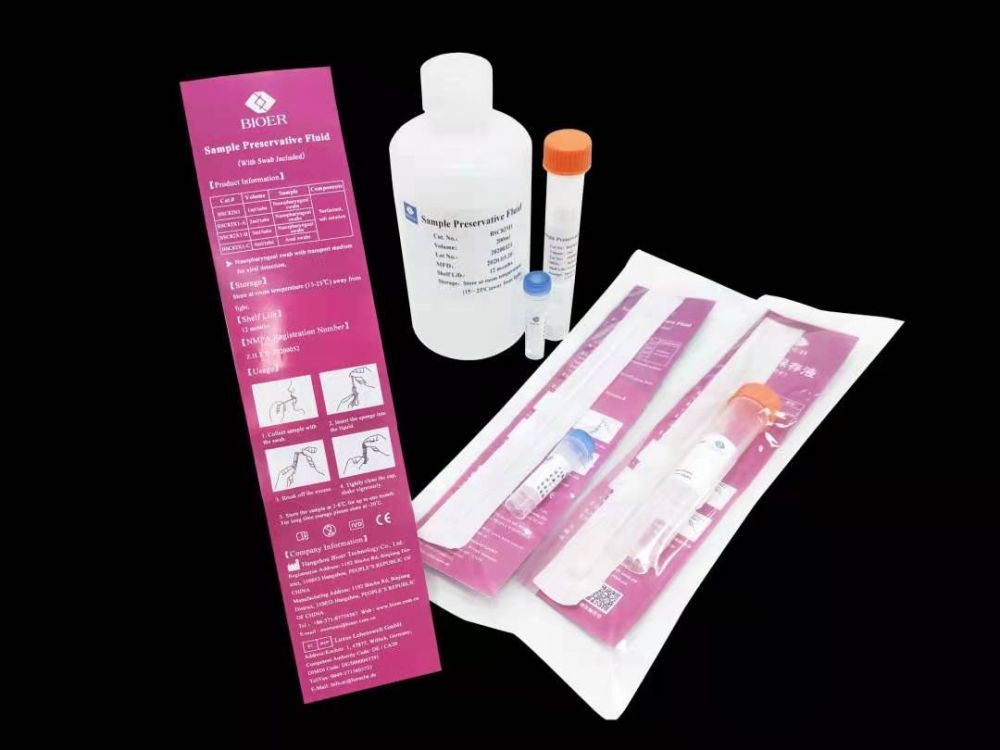
Sample preservation solution
This product is suitable for stable preservation of viral nucleic acids in nasopharyngeal swabs, anal swabs, tissues, feces, saliva, whole blood, serum, plasma, alveolar lavage fluid, pleural effusion and other samples. It can effectively preserve DNA / RNA virus nucleic acid for a long time and has stable performance; Fully considering the use environment, different specifications are set, which is convenient to use; It can be used for medical treatment, animal husbandry, inspection and quarantine, scientific research, etc.
stable
It contains high-efficiency DNase / RNase inhibitor, which can ensure the long-term stability of virus sample nucleic acid at room temperature and facilitate storage and transportation.
convenient
Various packaging specifications are provided for different scenarios, which can be used flexibly.
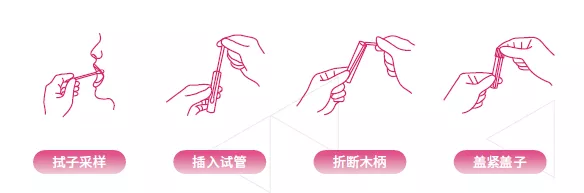
Operation process
Application case
Case 1:
Take throat swabs with different concentrations of influenza A virus as samples, and store the samples with this product. Test the storage stability of the samples stored at 4 ℃ and 37 ℃ for 7 days respectively. After RNA was extracted by Borg magabio virus DNA / RNA Purification Kit II, it was detected by real time RT-PCR. The comparison results of CT values are shown in Fig. 1 and Fig. 2:
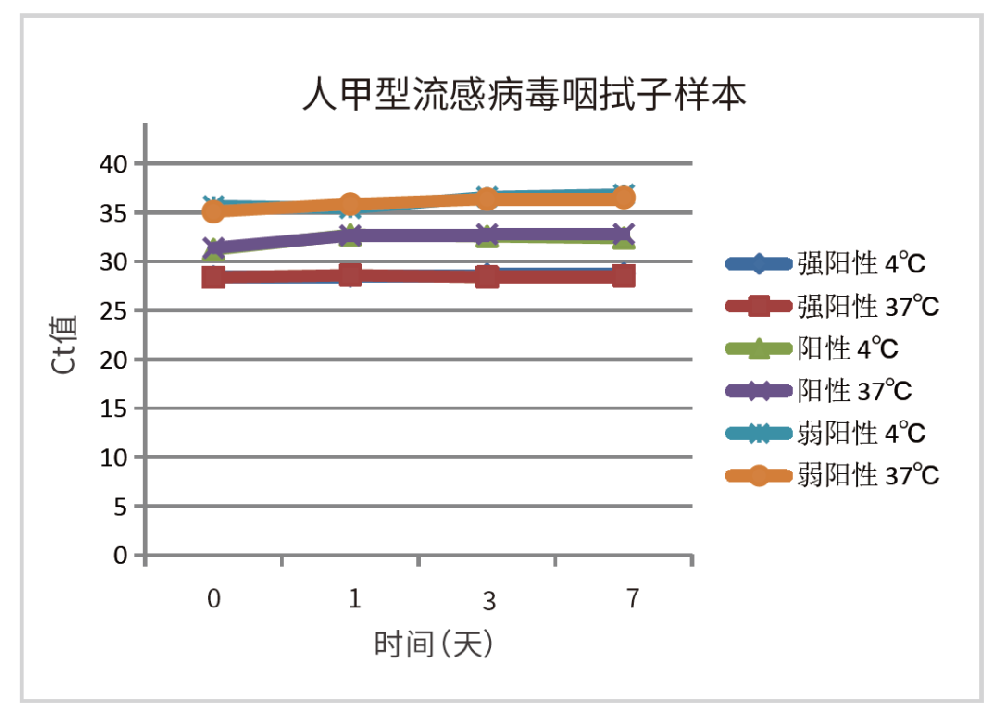
Figure 1
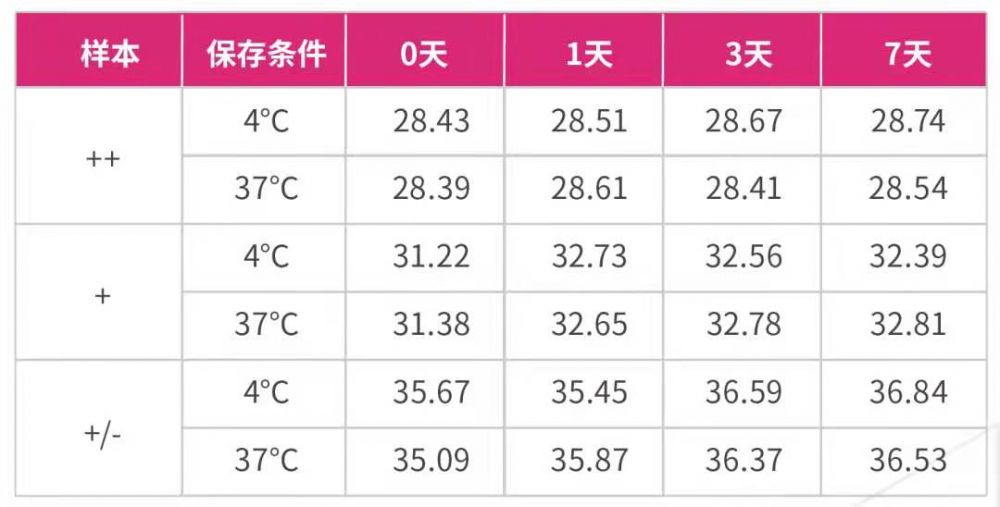
Figure 2
Case 2:
Take the feces positive for feline coronavirus (FCoV) as the sample, use the sample preservation solution in the ratio of 1:10 (1g feces is stored in 10ml preservation solution), compare the preservation stability of the feces stored at 4 ℃ and 37 ℃ for 7 days, extract FCoV RNA with Borge magabio virus DNA / RNA Purification kit II, and detect it by real time RT-PCR. The results are as follows 3 and 4:
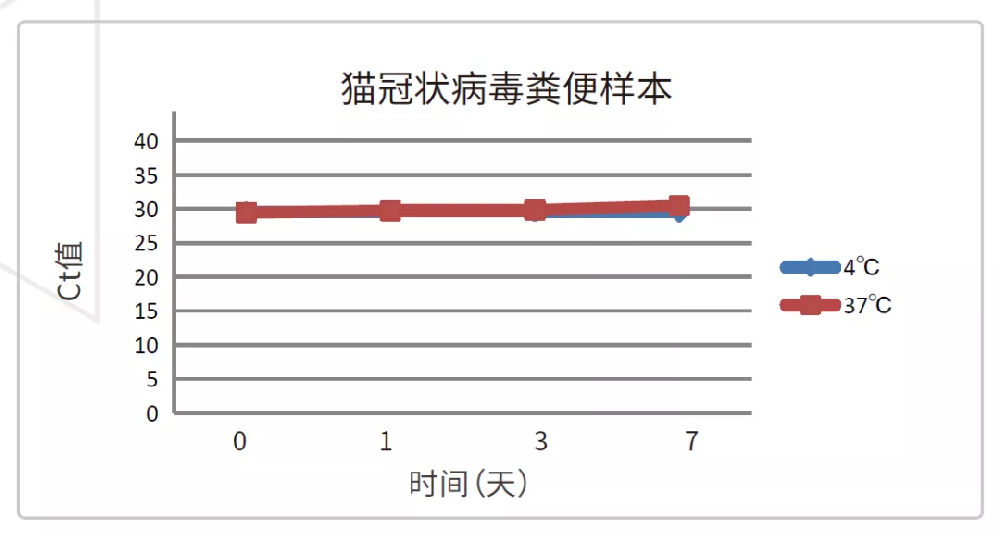
Figure 3
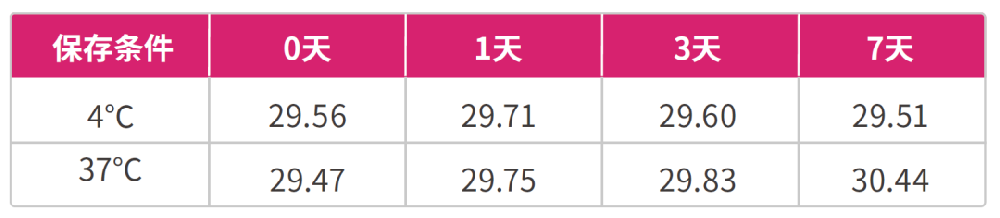
Figure 4
--> Return to list- Last:Qian Yujun, director of the market supervision and Administration Bureau of Hangzhou high tech Zone, and his delegation visited Bioer technology to listen to the situation, make suggestions and solve problems!
- Next:Industry benchmark! Bioer technology participates in the formulation of technical standards for shelter and mobile nucleic acid testing laboratories!

